HT-DESI-MS Facility: Capabilities
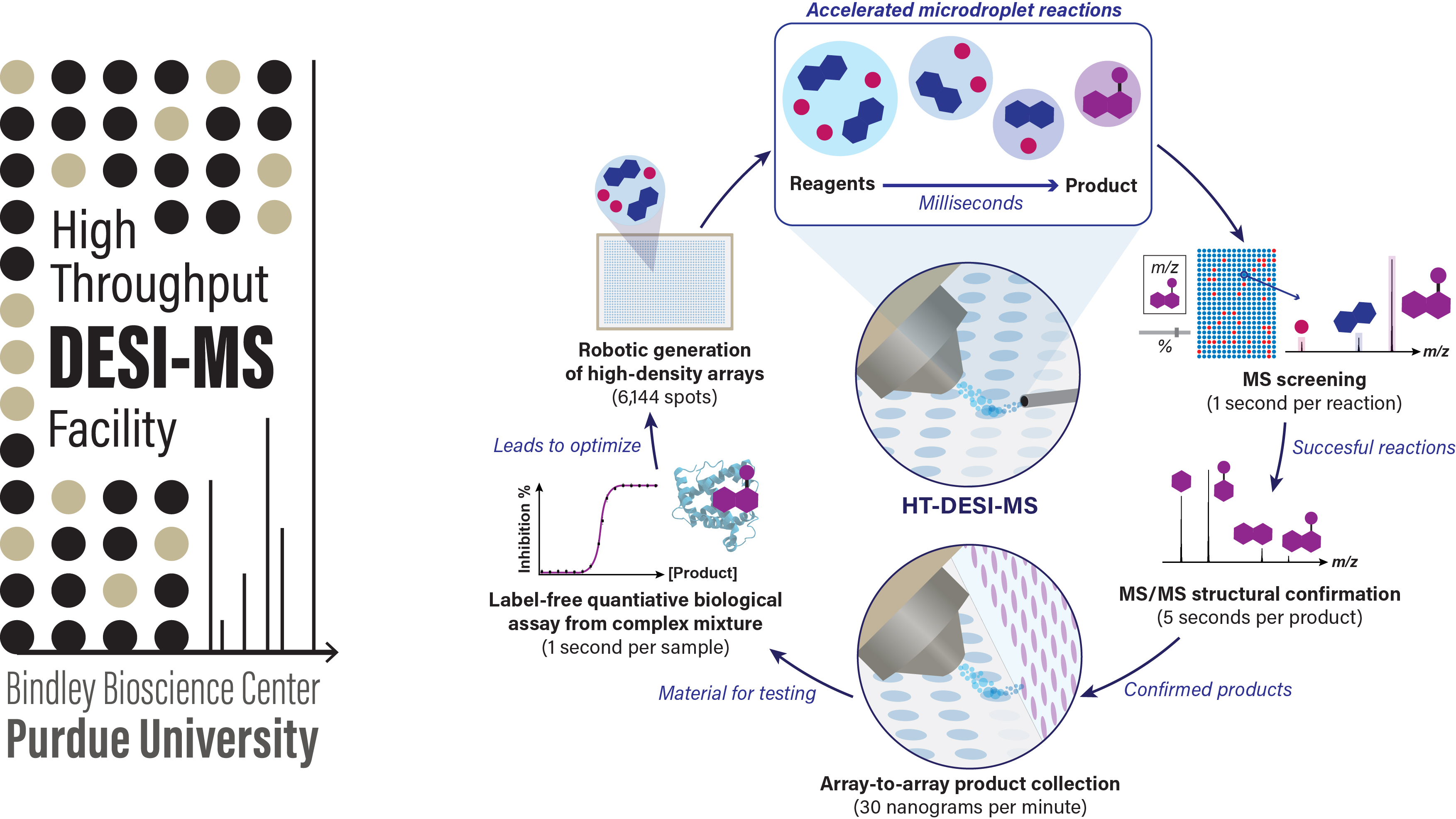
A wide variety of common organic reactions have been successfully explored via HT-DESI-MS. Multiple conventional array-based reaction formats (e.g., sealed heated plate reactors) are compatible with the platform, although the main advantage of the DESI technology results from leveraging accelerated droplet reactions to carry out both synthesis and analysis online (1 second per reaction) without any incubation time. Importantly, together with rapid qualitative and semi-quantitative determination of reaction outputs, structural confirmation of the reaction products is readily accessible via online tandem MS experiments on the same reaction mixtures (~6 seconds per reaction). A recent demonstration of the ultrafast chemical space exploration that is accessible using this technology can be found here.
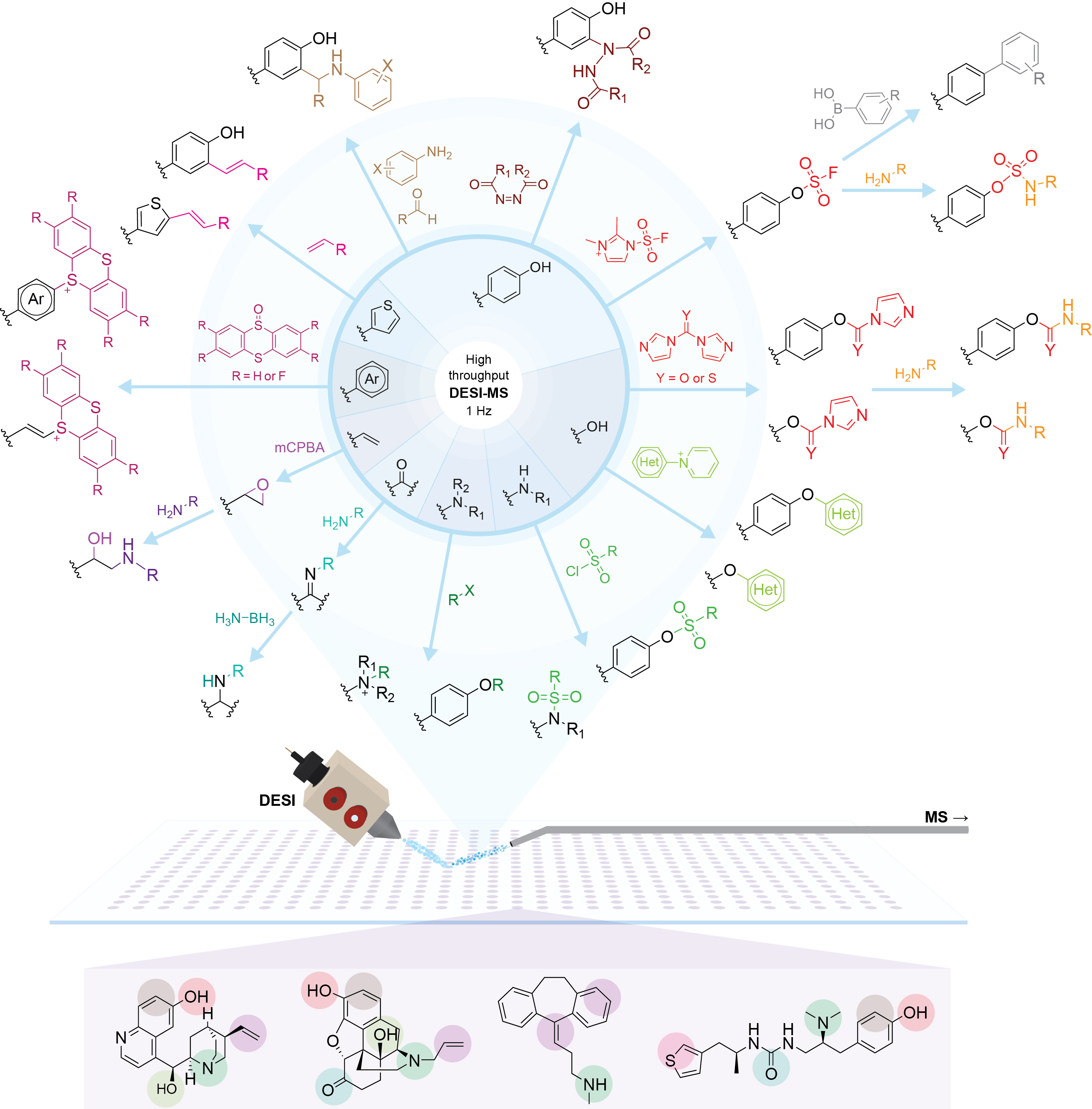
Some of the reactions that have been screened on the system include:
- Reductive amination
- Sulfonylation
- Alkenylation
- Ene-type click reactions
- Suzuki cross-coupling
- Aza-Michael reactions
- Mannich reactions
- Schiff base formation
- Thianthrenation
- SuFEx
- SnAr and SnAr-like catalyst-free C-N coupling
- Katritzky transamination
- Iso(thio)cyanate-based synthesis of heterocycles
Note that these transformations have been implemented both for combinatorial synthesis using simple scaffolds as well as for direct late-stage functionalization of already complex bioactive molecules.
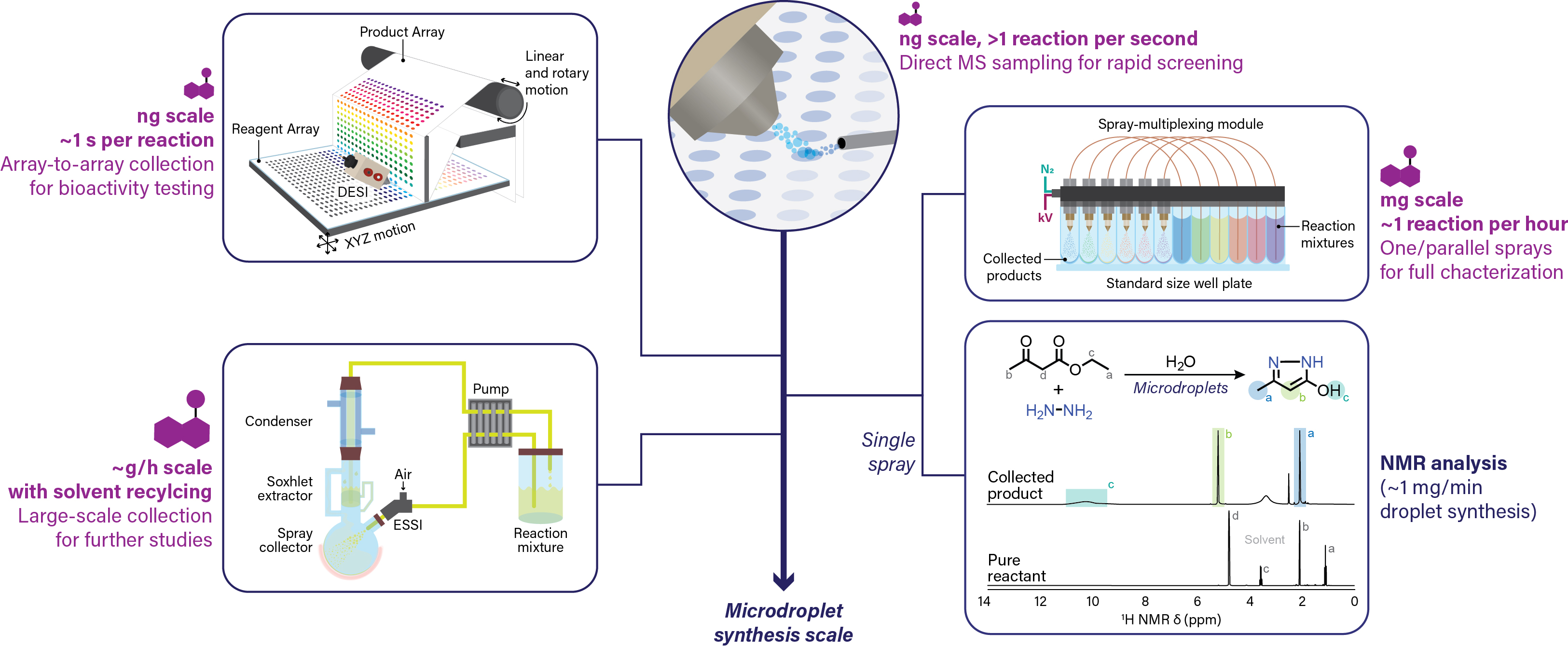
Collection of the DESI microdroplets (instead of MS sampling) effectively leads to a high-throughput methodology for nanogram scale synthesis. Automation of this process through a DESI-based array-to-array transfer system provides ready-to-use arrays of new chemical entities in quantities sufficient for a downstream biochemical assay. This prototype technology has been integrated as a module of the Gen 2.0 HT-DESI-MS platform. Its current synthetic efficiency is ~1 ug per minute, with the reaction time being customizable depending on the desired product yield.
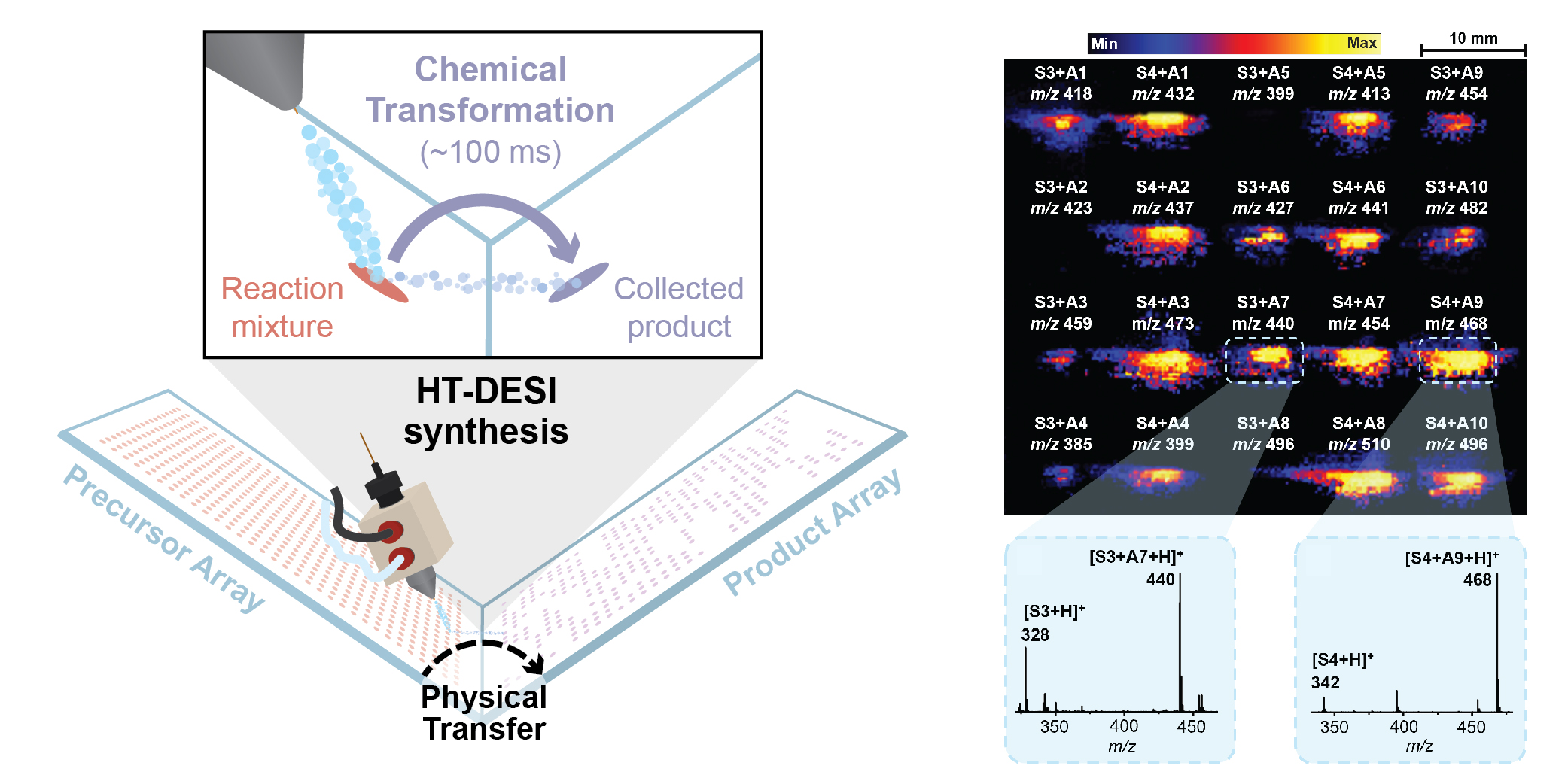
If larger amounts of material are needed for orthogonal analysis (e.g. NMR) or further downstream bioactivity evaluation (e.g. dose-response studies, cell-based confirmation), they are readily accessible through a variety of droplet-based scale-up methodologies as shown below. Note that the HT nanoscale synthesis approach is intended for direct-to-biology applications, i.e. the crude reaction product is utilized for bioactivity assessment without any purification.
HT-DESI-MS provides unique advantages for rapid and workup-free analysis of biological samples due to its inherent contactless operation and the online microextraction event involved in the DESI process. These features confer DESI with extraordinary tolerance for complex matrices containing high concentrations of non-volatile buffers (e.g. phosphate), salts, and detergents, all common components of bioassay matrices. Additionally, DESI can generate ions from objects rather than just solutions. In the context of bioanalysis, this means that it can be used to analyze a wide range of samples directly, including bacterial colonies, cell cultures and tissue biopsies. It is important to note that despite the complexicity of these types of samples, HT-DESI-MS provides excellent quantitative performance with a wide dynamic range and sensitivity down to femtogram levels of deposited material.
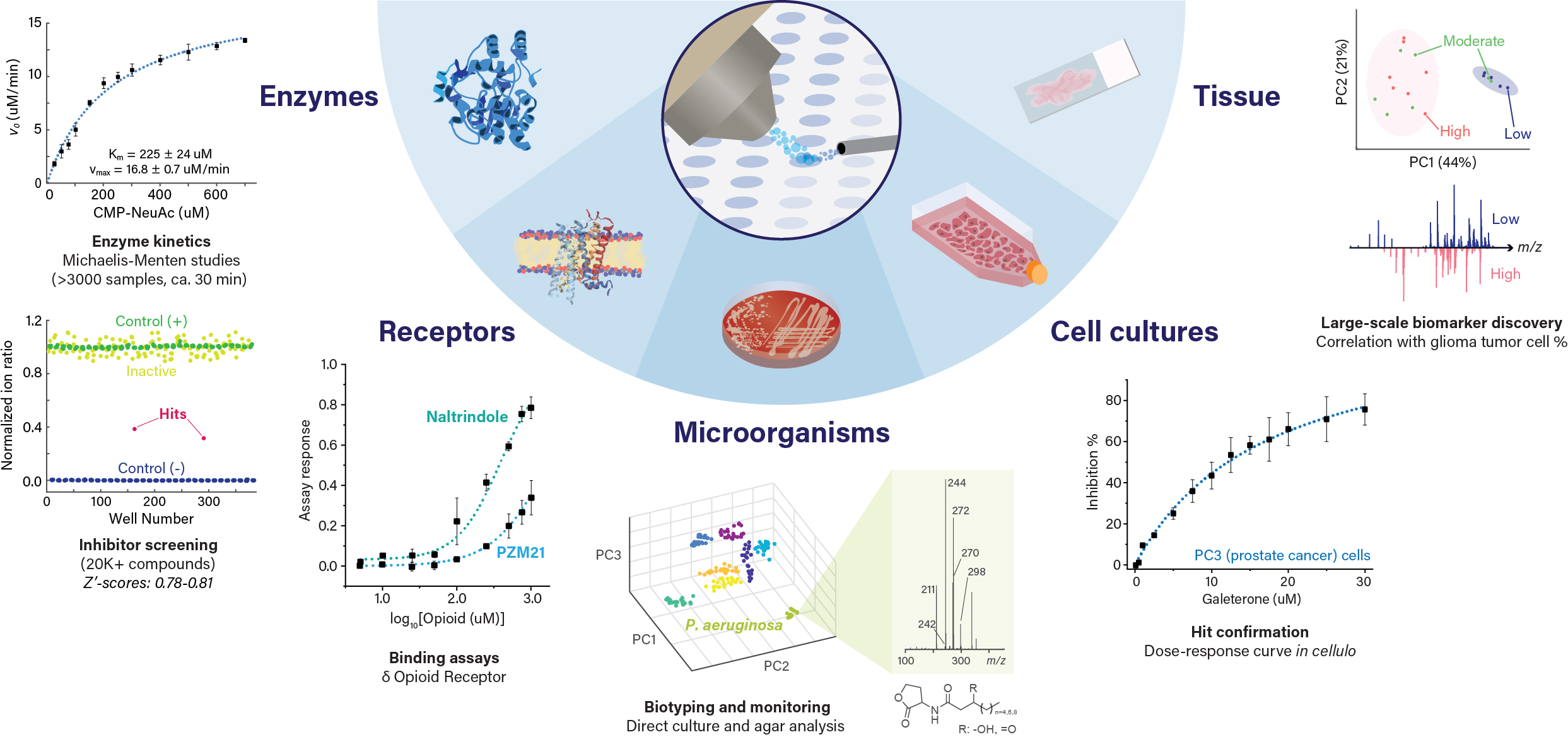
Enzymatic assays
A wide variety of enzymes have been studied using HT-DESI-MS. All these assays have been carried out in a label-free fashion (i.e. native substrates, no radioactive compounds, no coupled reactions) directly from the bioassay matrix. The abscence of labels makes DESI-based assays robust against typical interferences and expedites the assay development process. Quantitative results are obtained via reaction quenching and calculation of ion intensity ratios using the substrates and products or alternatively an internal standard.
HT-DESI-MS enzymatic assays have been utilized to (i) characterize the kinetic behavior of diseases targets as well as new enzymes and their mutants, (ii) high-throughput screening for hit identification and in vitro confirmation, (iii) directed evolution and protein engineering, and (iv) metabolism (multiple-enzyme) monitoring. Some of the enzymes explored include:
- Cholinesterases
- Lactamases
- Sulfotransferases
- Serine proteases
- Peptide cyclases
- SAH hydrolases
- Small-molecule kinases
- Methyltransferases
- Phospholipases
- Halogenases
- Sialyltransferases
Receptor and transporter binding
HT-DESI-MS has been utilized to replace radioligands in binding assays. For instance, relative binding affinities to human recombinant opioid receptors have been successfully determined via competitive binding assays against the endogenous opioid peptide leucine enkephalin. This technology has also been utilized to characterize and screen modulators of transporters, such as the bile salt export pump, using vesicle models.
Biosample analysis
Microarrays of biological samples, such as tissue biopsies, can be generated and directly screened both in targeted and untargeted fashion using HT-DESI-MS. Targeted studies have been utilized for monitoring specific biomarkers towards sample classification, assessment of intervention effects, and study of metabolic pathways. Some of the target compounds explored include neurotransmitters, cyclic peptides, lipids, and nucleotides. Untargeted studies have been implemented for disease biomarker discovery and generation of classification models using large sample sets.
Cells and microorganisms
HT-DESI-MS allows for direct sampling of microbial colonies or liquid cultures without the need for extraction, providing a fast alternative for lipid and metabolite monitoring. This strategy has been utilized for directed evolution campaigns, bacteria biotyping and evaluation of antibiotic susceptibility, metabolic consequences of drug exposure, and natural product discovery.
Similarly, HT-DESI-MS enables label-free quantitative analysis of human cell cultures, a capability that has been implemented in knock-down studies as well as in cellulo drug candidate hit confirmation.
Internal
- R. Graham Cooks (James Tarpo Jr. and Margaret Tarpo Department of Chemistry)
- Andrew Mesecar (Department of Biochemistry and Purdue Institute for Cancer Research)
- Vikki Weake (Department of Biochemistry)
- David Thompson (James Tarpo Jr. and Margaret Tarpo Department of Chemistry)
- Ramaswamy Subramanian (Bindley Bioscience Center and Department of Biological Sciences)
- Tillman Kubis (Elmore Family School of Electrical and Computer Engineering)
- Betsy Parkinson (James Tarpo Jr. and Margaret Tarpo Department of Chemistry)
- Jason Cannon (College of Health and Human Sciences)
- Raluca Ostafe (Purdue Institute of Inflammation, Immunology and Infectious Diseases)
- Young Jeong (Department of Industrial and Molecular Pharmaceutics)
- Daniel Flaherty (Borch Department of Medicinal Chemistry and Molecular Pharmacology)
- Mark Hall (Department of Biochemistry)
- John Tesmer (Department of Biological Sciences)
- Christina R. Ferreira (Bindley Bioscience Center and Department of Food Chemistry)
- Nicolás M. Morato (Purdue Institute for Cancer Research)
External Academic
- Jared Lewis (Indiana University)
- Kristina Brooks (University of Colorado)
Government
- National Center for Advancing Translational Sciences
- Forensic Science Center, Lawrence Livermore National Laboratory
- Frederick National Laboratory
- Naval Air Warfare Center Weapons Division
Industry
- J&J
- AbbVie
- Corteva
- Eli Lilly
- OnePot AI
Publications acknowledging the HT-DESI-MS facility (est. 2023)
- Cooks, R. G.; Feng, Y.; Huang, K.-H.; Morato, N. M.; Qiu, L. Re-Imagining Drug Discovery Using Mass Spectrometry. Isr. J. Chem. 2023, 63 (7–8), e202300034. DOI: 10.1002/ijch.202300034
- Huang, K.; Morato, N. M.; Feng, Y.; Cooks, R. G. High‐Throughput Diversification of Complex Bioactive Molecules by Accelerated Synthesis in Microdroplets. Angew. Chem. Int. Ed. 2023, 62 (22), e202300956. DOI: 10.1002/anie.202300956
- Feng, Y.; Morato, N. M.; Huang, K.-H.; Lin, M.; Cooks, R. G. High-Throughput Label-Free Opioid Receptor Binding Assays Using an Automated Desorption Electrospray Ionization Mass Spectrometry Platform. Chem. Commun. 2024, 60, 8224-8227. DOI: 10.1039/D4CC02346C.
- Li, J.; Morato, N. M.; Westover, L. S.; Abeywickrema, P.; Geng, J.; Piassek, M.; Harden, D.; Strambeanu, I.; Shi, Z.; Cooks, R. G.; Meng, J. High-Throughput Assessment of Bile Salt Export Pump Inhibition Using RapidFire-MS and DESI-MS. ACS Med. Chem. Lett. 2024, 15 (9), 1584-1590. DOI: 10.1021/acsmedchemlett.4c00302
- Huang, K.-H.; Morato, N. M.; Feng, Y.; Toney, A.; Cooks, R. G. Rapid Exploration of Chemical Space by High-Throughput Desorption Electrospray Ionization Mass Spectrometry. J. Am. Chem. Soc. 2024, 146 (48), 33112–33120. DOI: 10.1021/jacs.4c11037
- Ghosh, J.; Morato, N. M.; Feng, Y.; Cooks, R. G. High‐Throughput Drug Derivatization and Bioassay by Desorption Electrospray Ionization Mass Spectrometry. ChemPlusChem 2025, 2500164. DOI: 10.1002/cplu.202500164
- Huang, K.-H.; Chen, K.; Morato, N. M.; Sams, T. C.; Dziekonski, E. T.; Cooks, R. G. High-Throughput Microdroplet-Based Synthesis Using Automated Array-to-Array Transfer. Chem. Sci. 2025, 16 (17), 7544–7550. DOI: 10.1039/D5SC00638D
- Kim, A.; Morato, N. M.; Saha, P.; Eyimegwu, P.; Niyaz, A. A.; Huang, R.; Cooks, R. G.; Phelan, R. M.; Lewis, J. Selective Phosphorylation of Phenols and Benzenediols by the Kinase PsiK and Variants Thereof. Angew. Chem. Int. Ed. 2023, e202503538. DOI: 10.1002/anie.202503538
- Morato, N. M.; Mason, K. E.; Corzett, T. H.; Valdez, C. A.; Alfaro, T. M.; Hok, S.; Cooks, R. G.; Mayer, B. P. Accelerating countermeasure candidate discovery for A-series chemical warfare agent exposure. Proc. Natl. Acad. Sci. U.S.A. 2025, 122 (29), e2512471122. DOI: 10.1073/pnas.2512471122
- Stanhope, S. C.; Singhal, K.; Morato, N. M.; Feng, Y.; Meng, G.; Marlin, M. N.; Kotanko, C. C.; Jarrett, M. M.; Mesecar, A. D.; Cooks, G. R.; Weake, V. M. Drosophila Ahcy is a redox sensor that modulates gene expression to protect against light stress-induced retinal degeneration. Proc. Natl. Acad. Sci. U.S.A. 2025. DOI: 10.1073/pnas.2511388122
- Huang, K.-H.; Narendra, N.; Yap, K.; Morato, N. M., Chen, K.; Feng, Y.; Cooks, R. G.; Kubis, T.; Ferreira, C. R. High-Throughput Small-Scale Platform for Synthesis, Characterization, and Modeling of Per- and Polyfluoroalkyl Substances Analogs. Environ. Sci. Technol. Lett. 2025. DOI: 10.1021/acs.estlett.5c00699
- Ali, M. M.; Caruso, J. V.; Bushman, L.; Ellison, L.; Anderson, P. L.; Chohan, H.; Brooks, K. M.; Cooks, R. G. Rapid Selective Detection of Phosphatidylethanols (PEths) by Neutral Loss Scans in Tandem Mass Spectrometry to Evaluate Alcohol Consumption. Anal. Chem. 2025. DOI: 10.1021/acs.analchem.5c04552
- Li, J.; Aikonen, S.; Morato, N. M.; Hao, B.; Shi, Z; Strambeanu, I.; Cooks, R. G. Catalyst-free C-N Coupling under Ambient Conditions via High-Throughput Microdroplet Reactions. J. Org. Chem. 2025. DOI: 10.1021/acs.joc.5c02022
- Ghosh, J.; Morato, N. M.; LeFever, W. A.; Cooks, R. G. Accelerated and Green Synthetic Approach of N, S- and N, O Heterocyclics in Microdroplets. J. Am. Chem. Soc. 2026. DOI: 10.1021/jacs.5c12522
- Li, J.; Morato, N. M.; Hao, N.; Chen, A.; Jouffroy, L.; Kim, H.; Shi, Z.; Goossens, K.; Strambeanu, I. I.; Cooks, R. G. High-Throughput Chiral Analysis using Automated Desorption Electrospray Ionization Mass Spectrometry. Chem. Comm. 2026. DOI: 10.1039/D5CC06641G
Prior publications describing applications of the HT-DESI-MS technology
Reaction screening and optimization
- K. H. Huang, J. Ghosh, S. Xu, and R. G. Cooks. Late-Stage Functionalization and Characterization of Drugs by High-Throughput Desorption Electrospray Ionization Mass Spectrometry. ChemPlusChem 2022, e202100449.
- G. Murbach-Oliveira, K. Banerjee, M. M. Nociari, and D. H. Thompson. Continuous Flow Synthesis of A2E Guided by Design of Experiments and High-Throughput Studies. ACS Bio & Med Chem Au 2022, 2 (3), 297-306
- H. S. Ewan, S. A. Biyani, J. DiDomenico, D. Logsdon, T. J. P. Sobreira, L. Avramova, R. G. Cooks, and D. H. Thompson. Aldol Reactions of Biorenewable Triacetic Acid Lactone Precursor Evaluated Using Desorption Electrospray Ionization Mass Spectrometry High-Throughput Experimentation and Validated by Continuous Flow Synthesis. ACS Combinatorial Science 2020, 22 (12), 796-803.
- S. A. Biyani, Q. Qi, J. Wu, Y. Moriuchi, E. A. Larocque, H. O. Sintim, and D. H. Thompson. Use of high-throughput tools for telescoped continuous flow synthesis of an alkynylnaphthyridine anticancer agent, HSN608. Organic Process Research & Development 2020, 24 (10), 2240-2251.
- D. L. Logsdon, Y. Li, T. J. P. Sobreira, C. R. Ferreira, D. H. Thompson, and R. G. Cooks. High-throughput screening of reductive amination reactions using desorption electrospray ionization mass spectrometry. Organic Process Research & Development 2020, 24 (9), 1647-1657.
- Z. Jaman, D. L. Logsdon, B. Szilágyi, T. J. P. Sobreira, D. Aremu, L. Avramova, R. G. Cooks, and D. H. Thompson. High-throughput experimentation and continuous flow evaluation of nucleophilic aromatic substitution reactions. ACS Combinatorial Science 2020, 22 (4), 184-196
- B. P. Loren, H. S. Ewan, L. Avramova, C. R. Ferreira, T. J. P. Sobreira, K. Yammine, H. Liao, R. G. Cooks, and D. H. Thompson. High throughput experimentation using DESI-MS to guide continuous-flow synthesis. Scientific Reports 2019, 9 (1), 14745.
- P. W. Fedick, K. Iyer, Z. Wei, L. Avramova, G. O. Capek, and R. G. Cooks. Screening of the Suzuki cross-coupling reaction using desorption electrospray ionization in high-throughput and in Leidenfrost droplet experiments. Journal of The American Society for Mass Spectrometry 2019, 30 (10), 2144-2151.
- Z. Jaman, T. J. P. Sobreira, A. Mufti, C. R. Ferreira, R. G. Cooks, and D. H. Thompson. Rapid On-Demand Synthesis of Lomustine under Continuous Flow Conditions. Org. Process Res. Dev. 2019, 23, 334– 341.
Biosample analysis and label-free bioactivity assessment
- N. M. Morato, H. M. Brown, D. Garcia, E. H. Middlebrooks, M. Jentoft, K. Chaichana, A. Quiñones-Hinojosa, and R. G. Cooks. High-Throughput Analysis of Tissue Microarrays using Automated Desorption Electrospray Ionization Mass Spectrometry. Scientific Reports, 2022, 12, 18851.
- S. C. Kulathunga, N. M. Morato, Q. Zhou, R. G. Cooks, and A. D. Mesecar. Desorption Electrospray Ionization Mass Spectrometry Assay for Label-Free Characterization of SULT2B1b Enzyme Kinetics. ChemMedChem, 2022, 17, 9, e202200043.
- L. J. Szalwinski, L. E. Gonzalez, N. M. Morato, B. M. Marsh, and R. G. Cooks. Bacterial Growth Monitored by Two-Dimensional Tandem Mass Spectrometry. Analyst, 2022, 147, 5, 940-946.
- N. M. Morato, D. T. Holden, and R. G. Cooks. High-Throughput Label-Free Enzymatic Assays using Desorption Electrospray Ionization Mass Spectrometry. Angewandte Chemie International Edition, 2020, 59, 20459–20464.
Spectral library generation and big data
- M. T. Le, N. M. Morato, A. Kaerner, C. J. Welch, and R. G. Cooks. Fragmentation of Polyfunctional Compounds Recorded using Automated High-Throughput Desorption Electrospray Ionization. Journal of the American Society for Mass Spectrometry, 2021, 32, 8, 2261-2273.
Mechanistic Studies
- L. Qiu, N. M. Morato, K-H. Huang, and R. G. Cooks. Spontaneous Water Radical Cation Oxidation at Double Bonds in Microdroplets. Frontiers in Chemistry, 2022, 10, 903774.
- Y. Li, K.-H. Huang, N. M. Morato, and R. G. Cooks. Glass Surface as a Superbase: ‘Green’ Heterogeneous Catalyst and Degradation Reagent. Chemical Science, 2021, 12, 9816-9822.
Key background references on the HT-DESI-MS technology
HT-DESI-MS instrumentation and principles of operation
- N. M. Morato, M. T. Le, D. T. Holden, and R. G. Cooks. Automated High-Throughput System Combining Small-Scale Synthesis with Bioassays and Reaction Screening. SLAS Technology 2021, 26, 555– 571.
- M. Wleklinski, B. P. Loren, C. R. Ferreira, Z. Jaman, L. Avramova, T. J. P. Sobreira, D. H. Thompson, and R. G. Cooks. High Throughput Reaction Screening Using Desorption Electrospray Ionization Mass Spectrometry. Chemical Science 2018, 9, 1647– 1653.
- T. J. P. Sobreira, L. Avramova, B. Szilagyi, D. L. Logsdon, B. P. Loren, Z. Jaman, R. T. Hilger, R. S. Hosler, C. R. Ferreira, A. Koswara, D. H. Thompson, R. G. Cooks, and Z. K. Nagy. High-Throughput Screening of Organic Reactions in Microdroplets Using Desorption Electrospray Ionization Mass Spectrometry (DESI-MS): Hardware and Software Implementation. Analytical Methods 2020, 12, 3654– 3669.
- N. M. Morato and R. G. Cooks. Inter-platform assessment of performance of high-throughput desorption electrospray ionization mass spectrometry. Talanta Open 2021, 4, 100046.
DESI
- N. M. Morato and R. G. Cooks. Desorption electrospray ionization mass spectrometry: 20 years. Accounts of Chemical Research 2023, 56 (18), 2526-2536.
- Z. Takáts, J. M. Wiseman, B. Gologan, and R. G. Cooks. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473.
Reaction acceleration in microdroplets
- First description of the phenomenon: M. Girod, E. Moyano, D. I. Campbell, and R. G. Cooks. Accelerated bimolecular reactions in microdroplets studied by desorption electrospray ionization mass spectrometry. Chemical Science 2011, 2, 501–510.
- X. Yan, R. M. Bain, and R. G. Cooks. Organic reactions in microdroplets: reaction acceleration revealed by mass spectrometry. Angewandte Chemie International Edition 2016, 55 (42), 12960-12972.
- Z. Wei, Y. Li, R. G. Cooks, and X. Yan. Accelerated reaction kinetics in microdroplets: Overview and recent developments. Annual Review of Physical Chemistry 2020, 71 (1), 31-51.
- A popular tool for drug discovery just got 10 times faster. Purdue University News.
- Purdue chemists use DESI-MS to explore chemical space, accelerate reactions and impact drug discovery. Purdue University News.
- LLNL and Purdue University accelerate discovery of medical countermeasures for emerging chemical threats. Lawrence Livermore National Laboratory News.
- Safety threats revealed by mass spec. Purdue University News.
Hall for Discovery and Learning Research
207 S. Martin Jischke Dr. - Room 415
Purdue University
West Lafayette, IN 47907
