Accelerated Reactions in Confined Volumes
What is accelerated reactions in confined volumes?
The study of microdroplet and thin film reactions originated from early work in derivatization by reactive desorption electrospray ionization (reactive DESI). Here, it was observed that reactions which typically took minutes to hours in bulk solution occurred in the few seconds between ion generation and ion capture by a mass spectrometer. Many reactions have now been found to be accelerated by factors of 10 to 106 in microdroplets and thin films. Microdroplets, referring broadly to droplets in the aerosols created by electrospray and nebulization as well as atmospheric aerosols, emulsions, and Leidenfrost droplets, can be treated as a special reactor in which reactants are restricted in a limited space, giving rise to enhanced reaction rates. Similarly, thin films and micro-thin films are another form of confined volume reactors which exhibit modest reaction acceleration. Thin films allow for reactions to run for minutes to hours in comparison to the relatively short times of microdroplet reactions.
Reactions in microdroplets
Microdroplet reactions have been observed, in some cases, to exhibit greatly enhanced reaction rates when compared to the same reaction in bulk solution. In electrospray ionization (ESI), charged microdroplets are generated which are then analyzed by mass spectrometry (MS). Early work shows that, by increasing the distance of the electrospray emitter from the mass spectrometer inlet, conversion of reactants into products can be observed, implying reactions are occurring within microdroplets. In addition to increasing total reaction time, droplets travelling through the air undergo desolvation. Desolvation enhances the reactivity of microdroplets by: (i) Concentrating the reaction solution, (ii) increasing the mass transfer efficiency inside the droplet, and (iii) Increasing the surface to volume ratio, effectively increasing the concentration of reactant molecules on the droplet surface, where they are expected to be most reactive. An example of the Hantzsch reaction accelerated by ESI is shown in Figure 1.
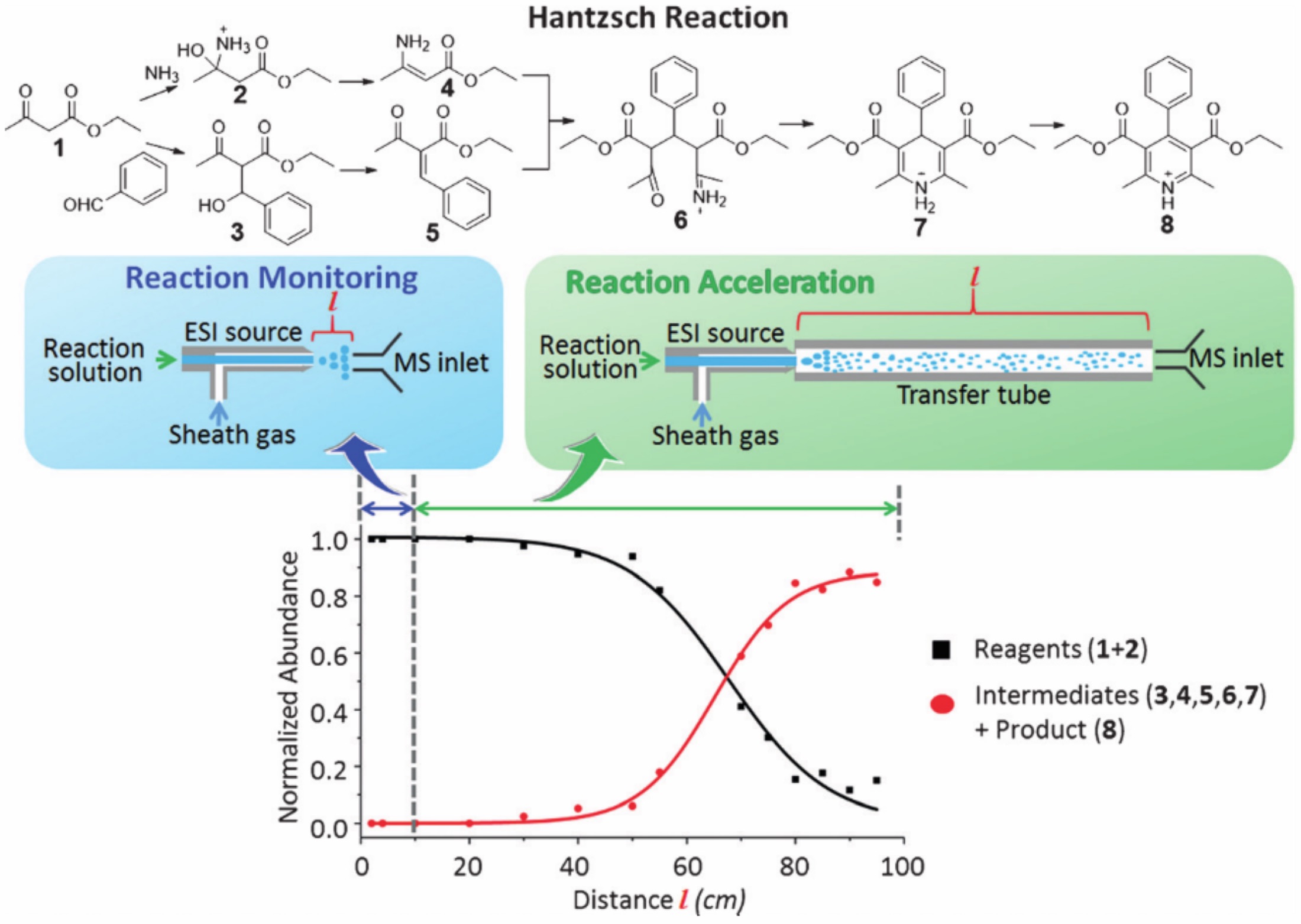
Figure 1. Function of reactant and product intensity vs. distance from spray source to MS inlet.
Measuring the size of electrospray droplets
The aim of the droplet microscopy experiments is to fundamentally understand droplets and therefore explore their utility. Although several instrumental techniques have been developed to probe droplet sizes, they fail in measuring droplet sizes with diameters of 400 nm or less mainly due to the diffraction limit of light. Hence a super resolution fluorescence microscopy-based method was developed to determine sizes of droplets below the diffraction limit. Rhodamine B, in different solvent compositions, was nanoelectroprayed and paper sprayed onto on conductive coverslip using a single voltage pulse. Images were collected using the 3D structured illumination microscopy (3D-SIM) mode and droplets sizes were determined using the circular Hough transformation program in MATLAB. In addition to studying droplets generated by different ionization sources, the effect of voltage, tip diameter and solvent composition was also studied. The smallest detectable droplets were found to have a diameter of 200nm. Future applications of this technique will involve the investigation of chemical reaction progression and acceleration in electrospray droplets.
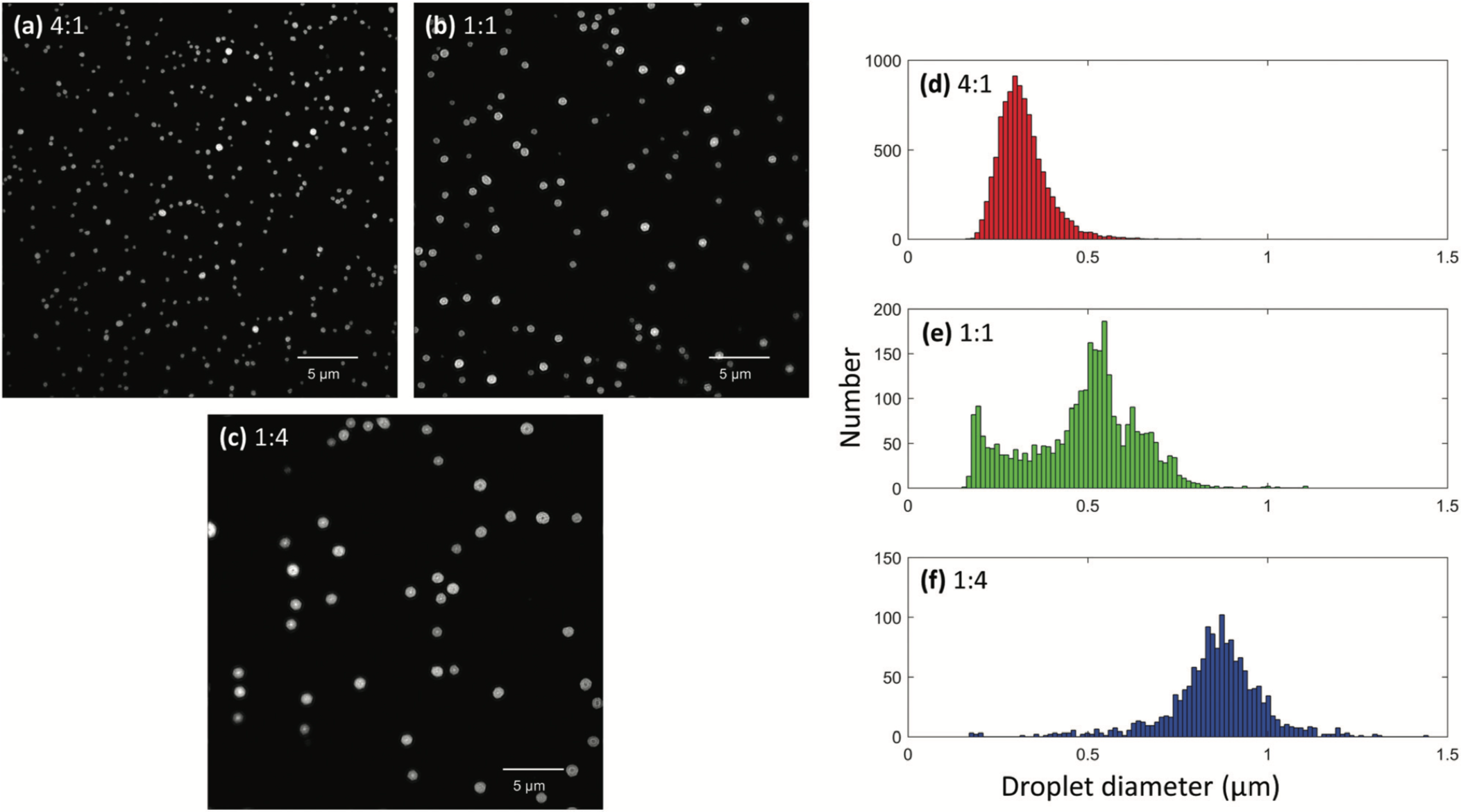
Figure 2. Super resolution microscopy images of nanoelectrosprayed droplets containing 1 mM rhodamine B and (a) 4:1, (b) 1:1, and (c) 1:4 ratios of methanol:glycerol. Corresponding droplet diameter distributions of (d) 4:1, (e) 1:1, and (f) 1:4 ratios of methanol:glycerol.
Thin film and microthin film reaction
Although very high reaction acceleration factors have been achieved in microdroplet reactions, their synthetic utility is limited due to the small amounts of product produced by such methods. Thin films are easily constructed by drop cast experiment and can be kept for minutes to hours’ time, allowing an accelerated reaction to go to completion. The early thin film reaction acceleration experiments were performed by drop casting reaction solutions to paper followed by paper spray analysis. For the Katritzky reaction, signals of the reactant species in MS were not observed in the case of the thin film reaction, implying essentially 100% conversion of reactants to products, while the product conversion ratio was less than 5% in bulk reaction after 10 minutes. To make even thinner films, both nonvolatile reactants and solvents are employed to construct the thin film. The protocol to construct a typical “microthin” film is shown in Figure 3. A nonvolatile amine (N,N-Dibutyl-1,3-propanediamine) was used to derivatize reducing sugars in a single cell extract followed by MS analysis. The success of using microthin films for single cell derivatization shows their potential as reaction systems for analysis of ultra-low volume samples.
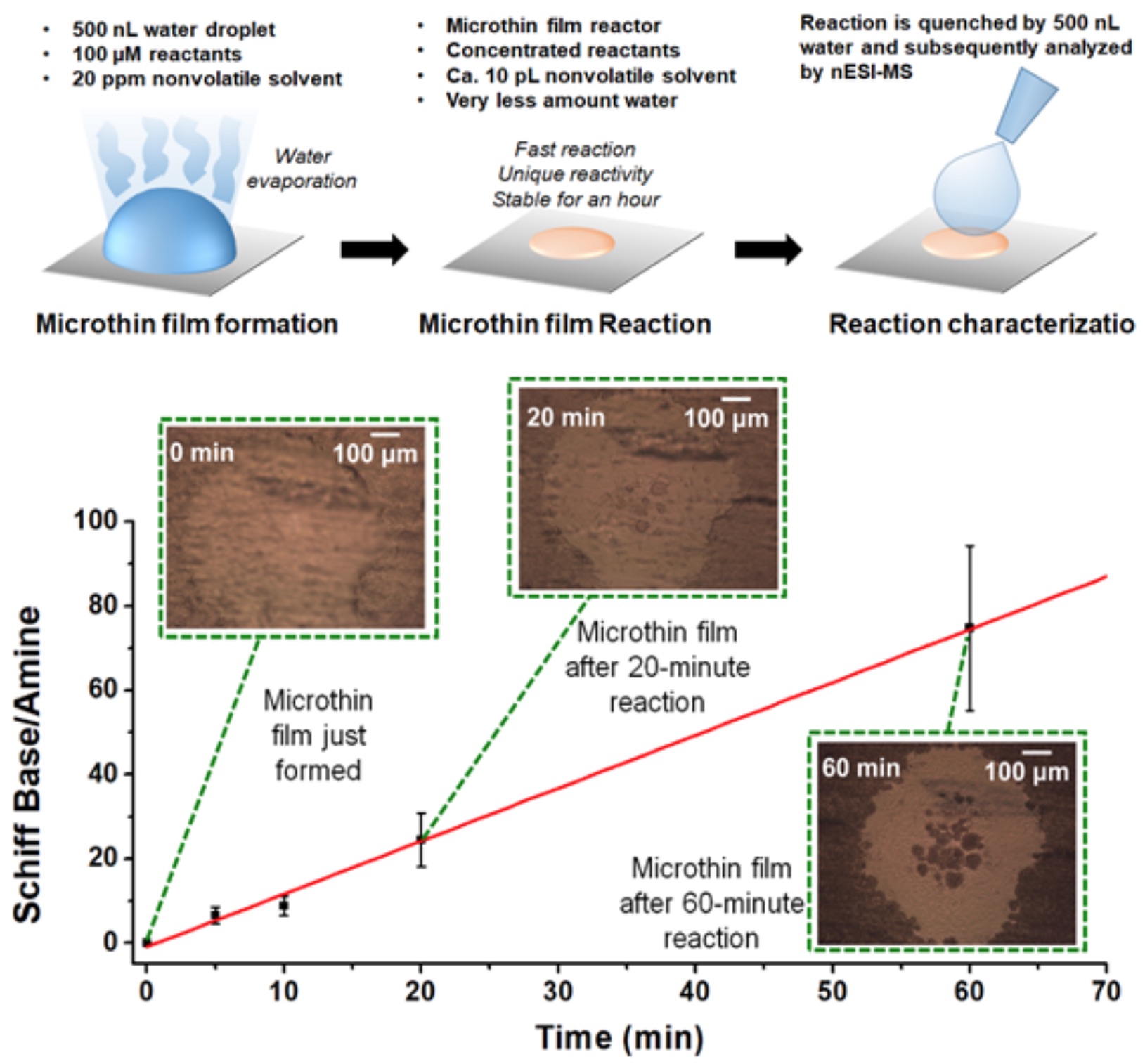
Figure 3. The construction of microthin film and the kinetics curve of imine formation reaction in the microthin films for hours.
Scale wise organic synthesis with continuous product deposition
By combining both thin films and microdroplets continuous deposition of a reaction mixture, high conversion ratios have been achieved. When microdroplets in an aerosol collide with the substrate a dynamic thin film is generated on the substrate (Figure 4). There is a tradeoff between reaction rate and conversion because the large reaction rate in droplets is discarded to form a dynamic thin film to obtain more products. It was found that the Claisen-Schmidt reaction between 6-hydroxyindanone with different substituted benzaldehydes can be accelerated 18 to 262 times relative to bulk reaction and the acceleration factor is independent of the reaction scale. Although this method is not as fast as microdroplet reaction and not as efficient as thin film reaction, the eclectic combination of microdroplet and thin film reaction is promising for synthetic use because of the flexibility in synthesis scale.
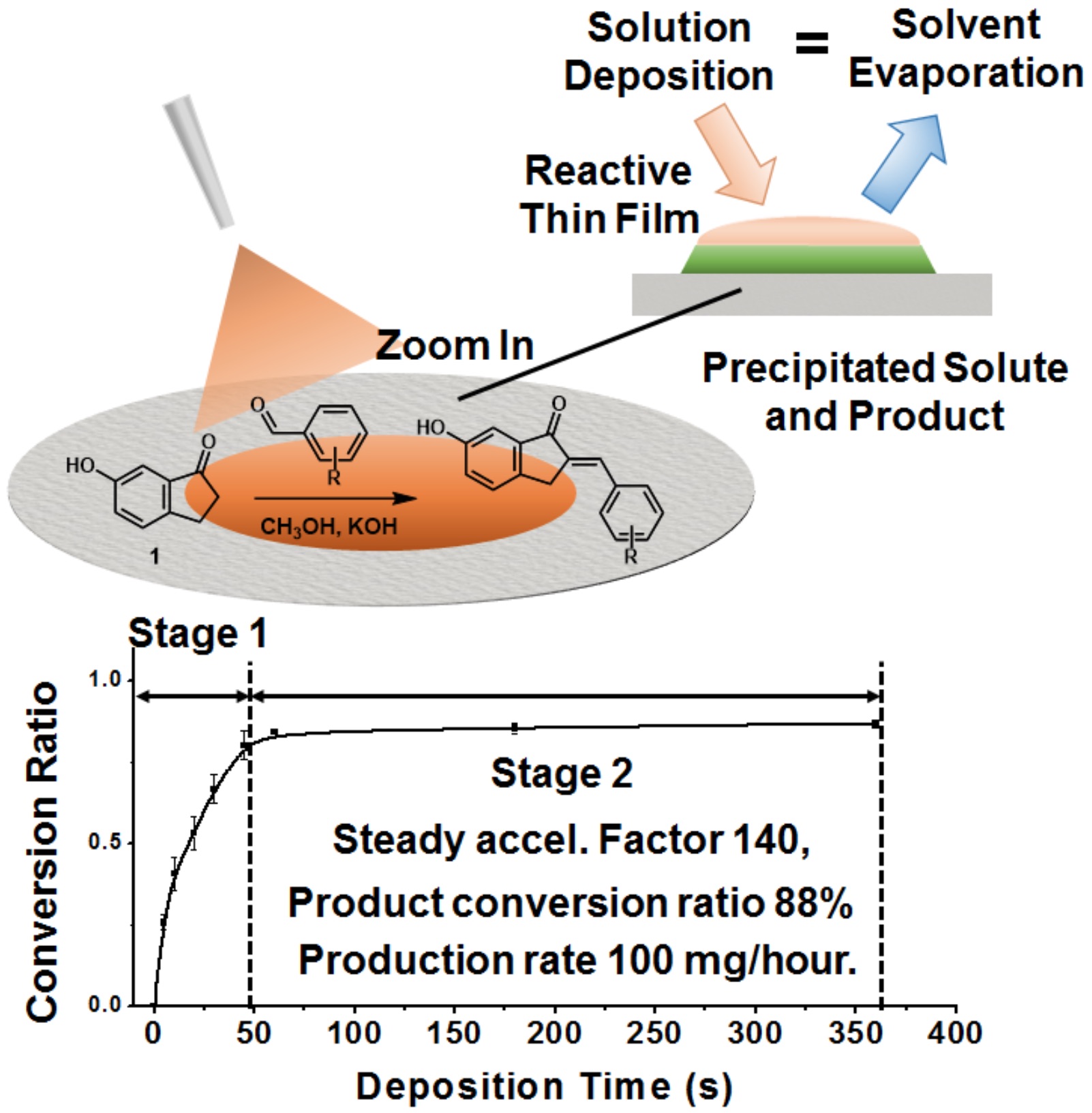
Figure 4. Thin films with continuous reactants deposition and product precipitation have been used for accelerated and scale up organic synthesis. Mechanistic study shows two stages of thin film reaction. Steady acceleration factor of 102and conversion ratio of 70 ~ 98% in diffusion controlled stage 2 enable us to achieve a rate of ca 100 mg/hour of Claisen-Schmidt reaction.
Unraveling chiraltiy transmission in by microdroplets reaction
Homochirogenesis, a set of events that can be encapsulated in three steps (symmetry breaking, chiral enrichment, and chiral transmission), is of prime importance to the origin of life. Amino acids are of particular interest in this respect for their biological significance. In particular, serine generates a magic-number cluster of exceptional abundance and high chiral purity, as revealed by mass spectrometric experiments. The strong chiral selectivity in this cluster suggests a possible connection to homochirality. Recently, we demonstrated the existence of the neutral serine octamer as a stable cluster, that its formation is highly chirally selective, and we suggest a structure analogous to that of Ser8H+. Proton transfer, isolation, and dissociation studies provide direct evidence for the existence of Ser8. Formation of the serine octamer requires either heating serine powder to sublimation, or microdroplet reactions, both of which have been suggested as reaction conditions for prebiotic chemistry.
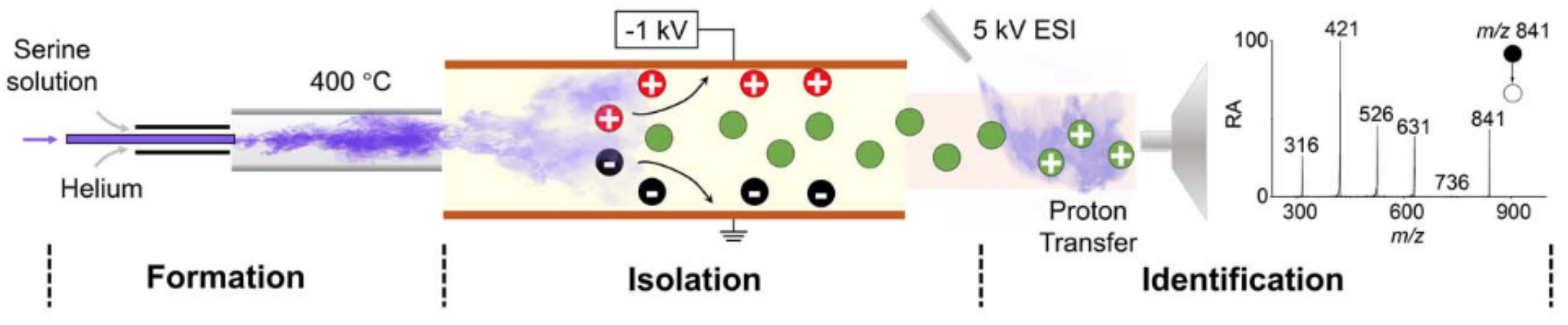
Figure 6. A nebulization method coupled with gentle on-line protonation and MS analysis is used to isolate and characterize the neutral serine octamer (Ser8). Ser8 was transmitted from the nebulizer into the atmospheric pressure ionization region while any residual protonated octamer was electrically deflected. Near-racemic serine when nebulized exhibited homochiral preference in the octamer and this we suggest is the origin of the homochiral preference of serine exhibited in Ser8H+.
Publications
- Müller, T.; Badu-Tawiah, A.; Cooks, R. G., Accelerated Carbon-Carbon Bond-Forming Reactions in Preparative Electrospray. Angew. Chem. Int. Ed. 2012, 51 (47), 11832-11835.
- Yan, X.; Bain, R. M.; Cooks, R. G., Organic Reactions in Microdroplets: Reaction Acceleration Revealed by Mass Spectrometry. Angew. Chem. Int. Ed. 2016, 55 (42), 12960-12972.
- Bain, R. M.; Pulliam, C. J.; Cooks, R. G., Accelerated Hantzsch electrospray synthesis with temporal control of reaction intermediates. Chem. Sci. 2015, 6 (1), 397-401.
- Hollerbach, A.; Logsdon, D.; Iyer, K.; Li, A.; Schaber, J. A.; Graham Cooks, R., Sizing sub-diffraction limit electrosprayed droplets by structured illumination microscopy. Analyst 2018, 143 (1), 232-240.
- Bain, R. M.; Pulliam, C. J.; Thery, F.; Cooks, R. G., Accelerated Chemical Reactions and Organic Synthesis in Leidenfrost Droplets. Angew. Chem. Int. Ed. 2016, 55 (35), 10478-10482.
- Li, Y.; Liu, Y.; Gao, H.; Helmy, R.; Wuelfing, W. P.; Welch, C. J.; Cooks, R. G., Accelerated Forced Degradation of Pharmaceuticals in Levitated Microdroplet Reactors. Chem. Eur. J. 2018, 24 (29), 7349-7353.
- Wei, Z.; Wleklinski, M.; Ferreira, C.; Cooks, R. G., Reaction Acceleration in Thin Films with Continuous Product Deposition for Organic Synthesis. Angew. Chem. Int. Ed 2017, 56 (32), 9386-9390.
- Wei, Z.; Zhang, X.; Wang, J.; Zhang, S.; Zhang, X.; Cooks, R. G., High yield accelerated reactions in nonvolatile microthin films: chemical derivatization for analysis of single-cell intracellular fluid. Chem. Sci. 2018, 9 (40), 7779-7786.
- Zhang, H.; Wei, Z.; Jiang, J.; Cooks, R. G., Nebulization Prior to Isolation, Ionization, and Dissociation of the Neutral Serine Octamer Allows Its Characterization. Angew. Chem. Int. Ed. 2018, 57, 17141-17145