Origins of Life
"Flower in the crannied wall, I pluck you out of the crannies, I hold you here, root and all, in my hand, Little flower—but if I could understand What you are, root and all, and all in all, I should know what God and man is." — Alfred, Lord Tennyson
Early Earth was a mosaic of dynamic chemical environments: from the seething depths of hydrothermal vents to the drying surfaces of hydrothermal pools, and the delicate aerosols born of sea sprays. Each of these settings provided unique conditions that could have fostered the chemical evolution necessary for life.
Our current research explores the novel field of microdroplet chemistry, representing small water aerosols. In these tiny droplets, reactions deemed impossible under bulk conditions become feasible. The microdroplet environment accelerates the formation of peptides from amino acids, offering a glimpse into how life's building blocks might have assembled in prebiotic times [1,2].
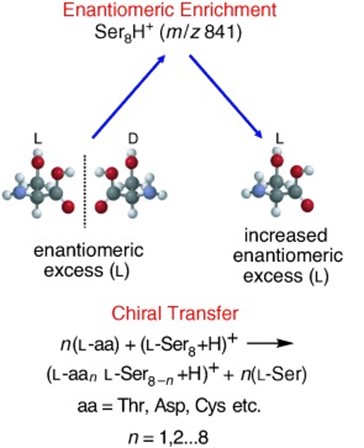
Chirality adds another layer to this intricate puzzle. Modern life predominantly utilizes L-amino acids and D-sugars, a preference that hints at a pivotal moment in chemical evolution. We investigate phenomena like the serine octamer—a molecular cluster exhibiting chiral preference and inducing an enantiomeric excess. This not only fascinates on its own but also underscores the significance of non-covalent interactions and molecular clusters in shaping chirality and influencing broader chemical processes [3–5].
Publications
-
Holden, D. T.; As, N.; Morato, M.; Cooks, R. G. Aqueous Microdroplets Enable Abiotic Synthesis and Chain Extension of Unique Peptide Isomers from Free Amino Acids. Proc Natl Acad Sci USA 2022, 119 (42), e2212642119. https://doi.org/10.1073/pnas.2212642119.
-
Qiu, L.; Cooks, R. G. Oxazolone Mediated Peptide Chain Extension and Homochirality in Aqueous Microdroplets. Proc Natl Acad Sci USA 2024, 121 (2), e2309360120. https://doi.org/10.1073/pnas.2309360120.
-
Cooks, R. G.; Zhang, D.; Koch, K. J.; Gozzo, F. C.; Eberlin, M. N. Chiroselective Self-Directed Octamerization of Serine: Implications for Homochirogenesis. Anal Chem 2001, 73 (15), 3646–3655. https://doi.org/10.1021/ac010284l.
-
Takats, Z.; Nanita, S. C.; Cooks, R. G. Serine Octamer Reactions: Indicators of Prebiotic Relevance. Angewandte Chemie International Edition 2003, 42 (30), 3521–3523. https://doi.org/10.1002/anie.200351210.
-
Nanita, S. C.; Cooks, R. G. Serine Octamers: Cluster Formation, Reactions, and Implications for Biomolecule Homochirality. Angewandte Chemie International Edition 2006, 45 (4), 554–569. https://doi.org/10.1002/anie.200501328.